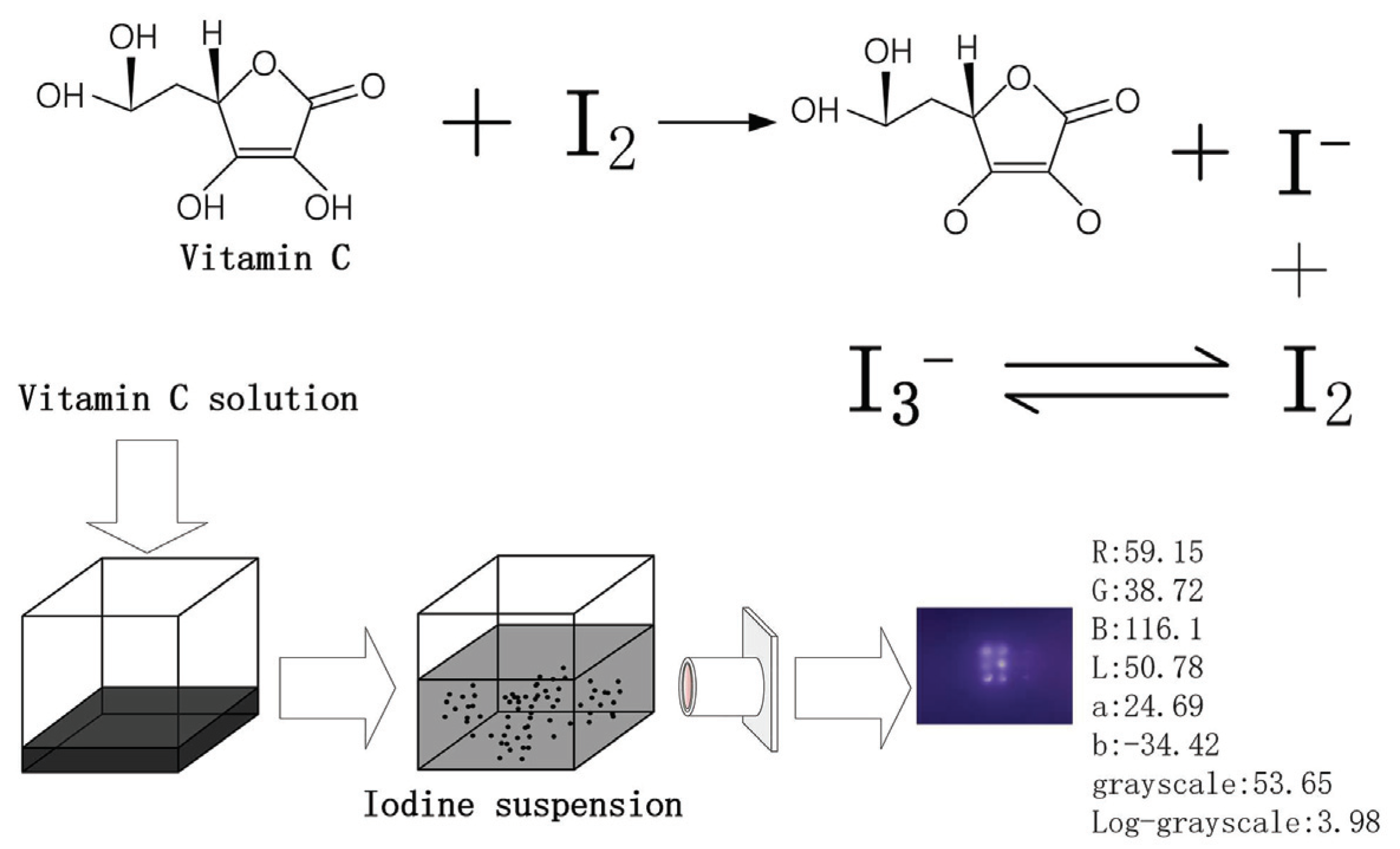
Applied Sciences | Free Full-Text | Determination of Vitamin C in Foods Using the Iodine-Turbidimetric Method Combined with an Infrared Camera

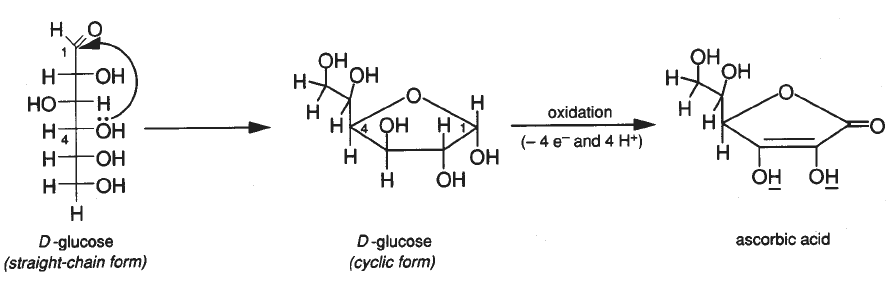
Ascorbic acid (vitamin C), along with having many other reputed properties, acts as an antioxidant. The following equation illustrates its antioxidant properties. H_2C_6H_6O_6 \to C_6H_6O_6 + H_2(g) What is an antioxidant? Assign
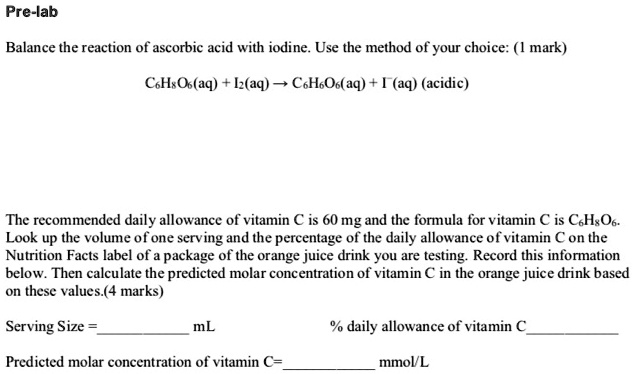
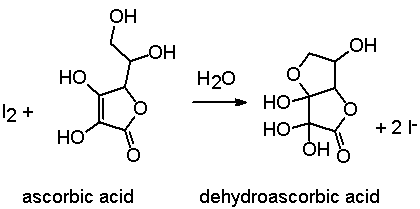
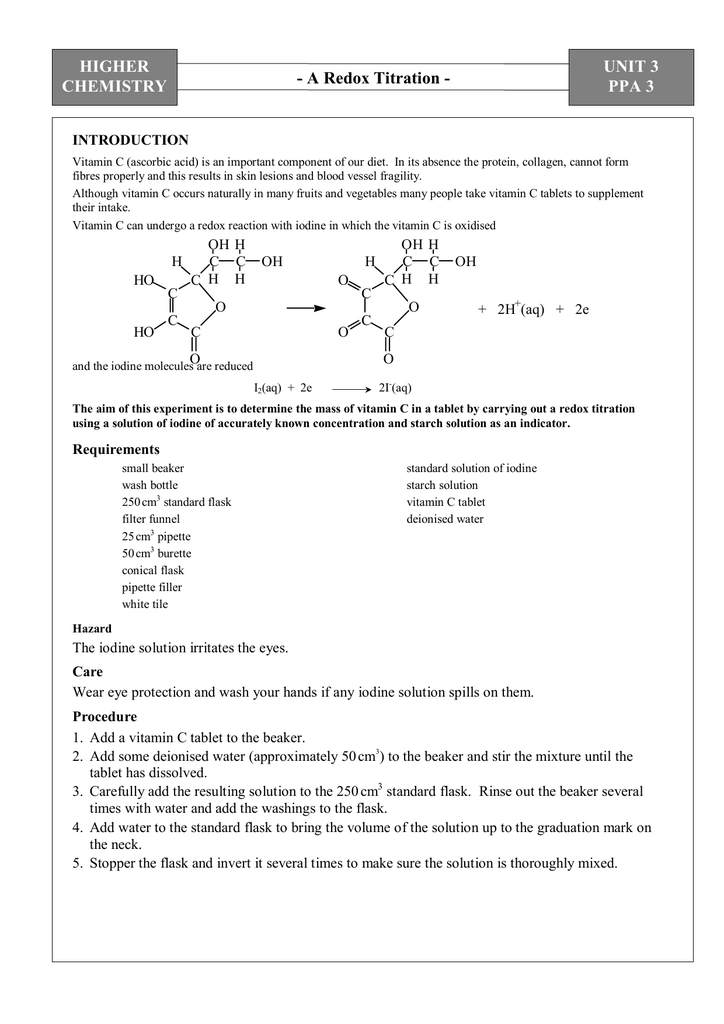
SOLVED: Pre-lab Balance the reaction of ascorbic acid with iodine. Use the method of your choice: mark) CoHsOs(aq) [z(aq) CoHsOs(aq) (aq) (acidic) The recommended daily allowance of' vitamin € is 60 mg
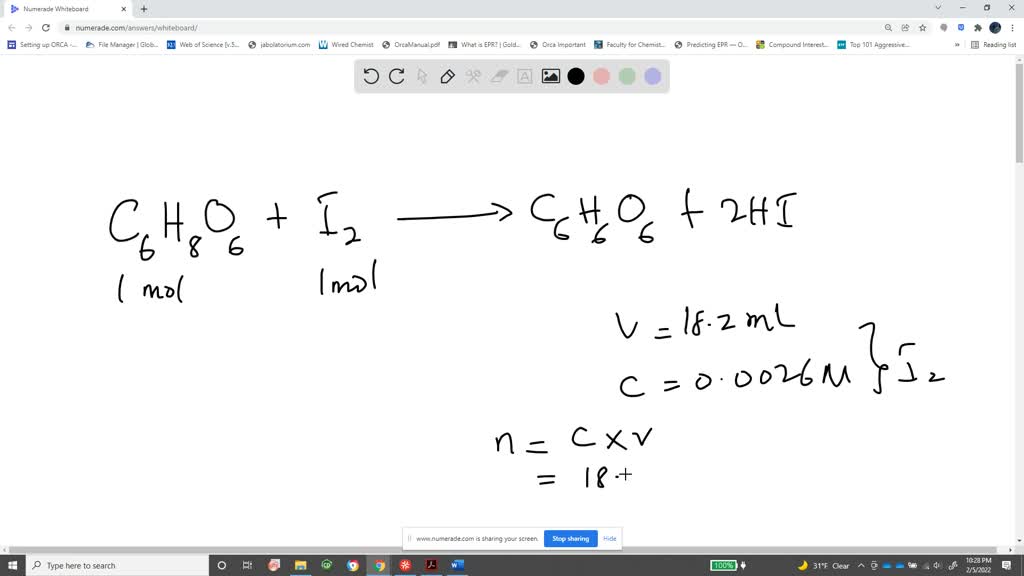
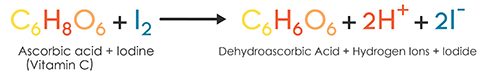
SOLVED: The titration of vitamin C with iodine proceeds according to the given equation. C6H8O6+I2⟶C6H6O6+2HI Suppose it takes 18.21 mL of 0.0026 M I2 solution to reach the end point of the

OneClass: 1. Ascorbic acid (vitamin C) in a juice drink can be determined by means of a constant-curr...